FULL STUDY TITLE
 Trial of Sequential Medications AfteR TNFi Failure in Juvenile Idiopathic Arthritis (SMART-JIA)
Trial of Sequential Medications AfteR TNFi Failure in Juvenile Idiopathic Arthritis (SMART-JIA)
|
The following information is intended for researchers and healthcare professionals who may wish to consider their possible involvement in the trial. A more concise and user-friendly informative section of the trial, specifically designed for patients and their families, is available on the trial website: Please download here the study poster. |
STUDY OVERVIEW
Brief Summary
This study is an open-label, randomized, multicenter trial that incorporates a multi-arm design comparing each of 3 non-TNFi (Tumor Necrosis Factor inhibitor) medications to a second TNFi (active control) within a sequential multiple assignment randomized trial design with 2 randomization stages corresponding with clinical decision points. The first randomization addresses whether each of the 3 non-TNFi medications is superior to treatment with a second TNFi. The second randomization allows identification of optimal sequential use of biologics (treatment strategies).
Detailed Description
The goal of the study is to provide an evidence base for selecting sequential medication(s) if a JIA patient fails initial bDMARD. SMART-JIA is a pragmatic, international, open-label, randomized trial comparing treatment with a second TNFi (active control) to each of 3 different medications (IL-6i, JAKi, or ABA) in children aged 2 to 17 years with pcJIA and inadequate response to initial TNFi. Leveraging sequential multiple assignment randomized trial (SMART) design methodology, we will implement a second randomization to assess the effectiveness of changing medication if there is inadequate response to the first study medication. This approach allows identification of optimal strategies for medication sequencing based on individual characteristics and provides critical insights to inform future studies.
SMART-JIA will study the efficacy of a second TNFi (active control) compared to each of 3 other already US Food and Drug Administration (FDA)-approved and European Union (EU)-approved non-TNFi medications currently used to treat pcJIA (IL-6i, JAKi, and ABA). TNFi, IL-6i, and ABA are administered by subcutaneous (SQ) injection weekly, or every other week, or every three weeks, and JAKi (e.g., tofacitinib) is taken orally twice daily. All study treatments have similar safety profiles and are standard of care (SOC) worldwide. This in addition to the pragmatic and full-scale nature of the trial will ensure its completion. Successful completion of this trial will substantially impact the clinical care and outcomes of children with pcJIA, shifting the current trial-and-error treatment paradigm to a smart, precise approach.
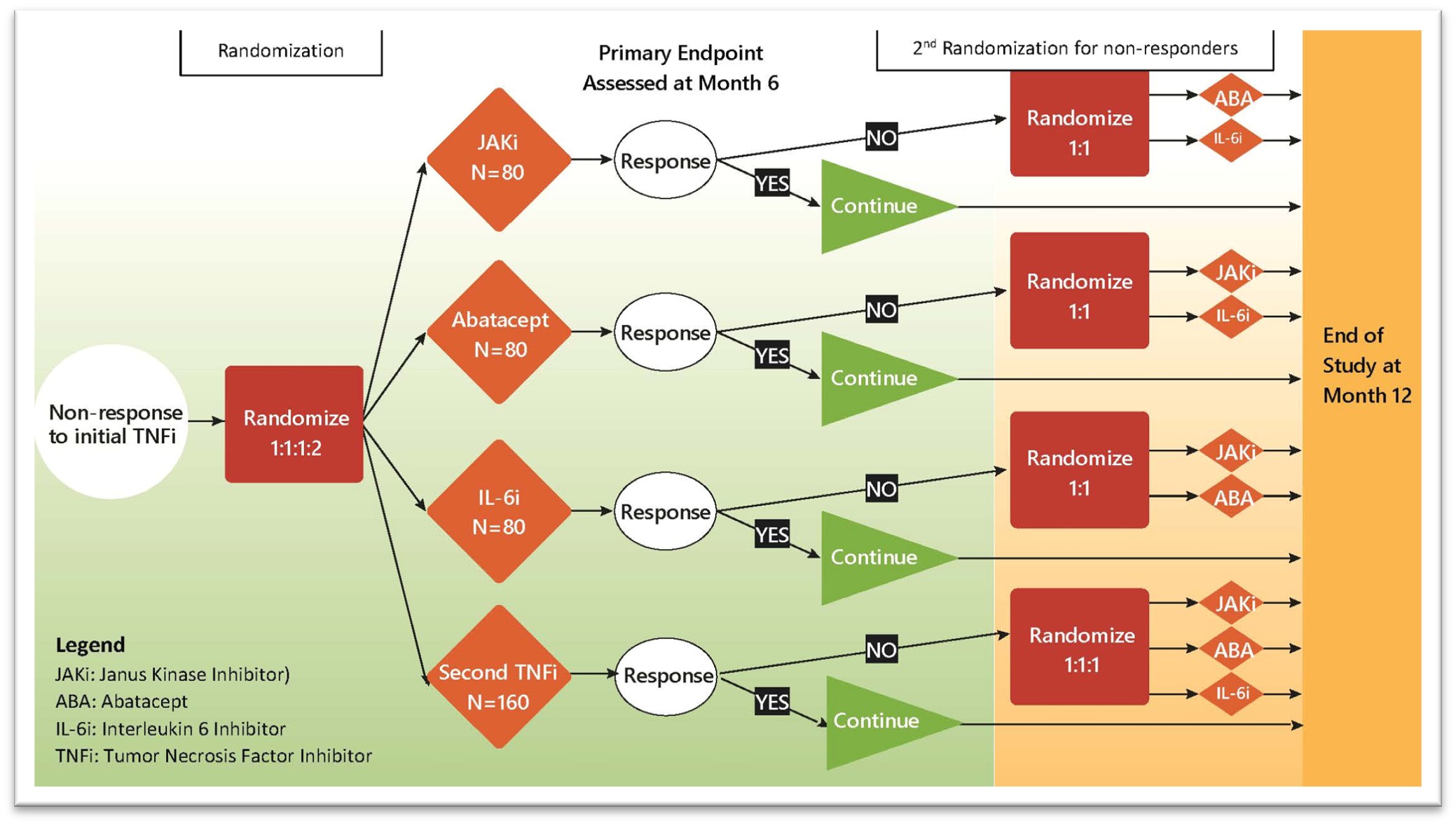
Figure 1: SMART-JIA Flow Diagram
Sponsor: Duke Clinical Research Institute (DCRI), 300 West Morgan St., Durham, NC 27701, USA
EU Clinical Trial number: 2025-520923-25
Principal Investigator (Contact PI)
Laura E. Schanberg, MD
Department of Pediatrics
Duke University Medical Center
laura.schanberg@duke.edu
International Principal Investigator
Fabrizio De Benedetti, MD, PhD
Head, Division of Rheumatology
Head, Laboratory of ImmunoRheumatology
Ospedale Pediatrico Bambino Gesù
debenedetti@opbg.net
More details on Clinicaltrials.gov at this link.
PReSTaR and the SMART-JIA trial
The PReS Network for Trial and Research (PReSTaR), as an independent academic clinical research network stemming from a collaboration of the Paediatric Rheumatology European Society (PReS) and IRCCS Istituto Giannina Gaslini (Italy), will act as the academic Contract Research Organization (CRO) for the SMART-JIA study. PReSTaR will support participating European Union sites throughout all phases of the trial and provide comprehensive scientific, medical, regulatory, and operational support, including protocol development and amendments, regulatory submissions, site selection and start-up, and coordination with National Competent Authorities, local Ethics Committees, and CTIS. PReSTaR will oversee site management and monitoring activities, ensure data quality, manage study drug distribution, and coordinate safety reporting. PReSTaR will also contribute to database and CRF development, statistical activities, training programs, and study governance meetings, ensuring high-quality trial execution and full compliance with Good Clinical Practice (GCP) across EU sites.
For more info, please contact PReSTaR@gaslini.org



